There is little doubt that medical technology has dramatically enhanced the quality and longevity of our lives. We continue to see innovations that not only aid in earlier detection of disease but that makes treatments more precise, effective and, in some cases, less arduous. Among these medical advances are wearable sensors which provide information to physicians while empowering individuals to participate in their own care.
"Mobile technology has become a ubiquitous part of everyday life and is changing the way we offer clinical care and perform clinical research," according to the National Library of Medicine.
"We have unprecedented access to data for one's self-care, as well as for sharing with health care providers."
But it is important to remember that while we continue to reap the benefits of medical innovation, technological devices do not always perform as intended.
Health Canada recently sent out an alert, stating that some FreeStyle Libre 3 Plus glucose monitor sensors may provide incorrect low glucose readings.
In an urgent medical device recall, Abbott Diabetes Care stated its FreeStyle Libre 3 Plus sensors "may provide incorrect low glucose readings, which if undetected may post a potential health risk for people living with diabetes." The issue affects a subset of FreeStyle Libre 3 Plus sensors but does not apply to the FreeStyle Libre 3 Plus reader and app or any other Libre product, according to Abbott.
Affected About Three Million Sensors.
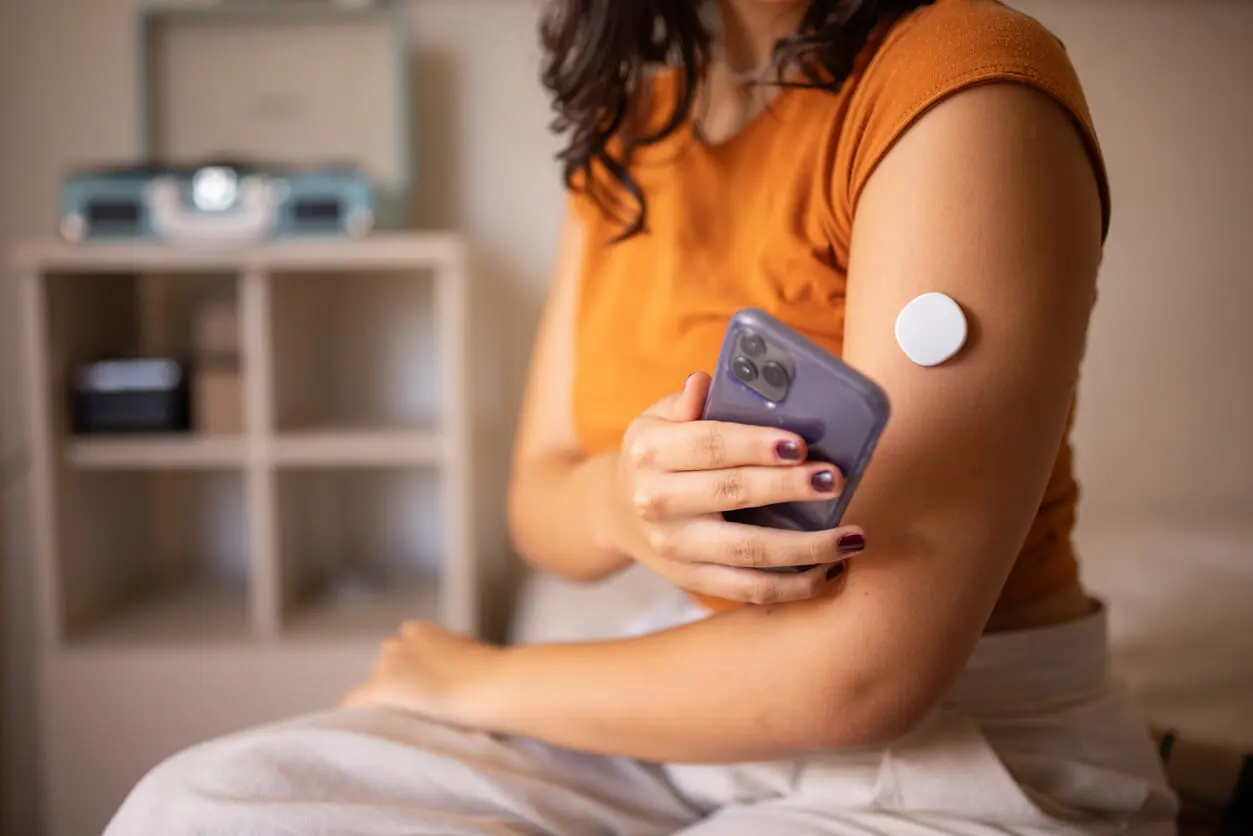
According to a CBC report, the sensors are "devices that measure glucose levels in fluid just beneath the skin to provide real-time measurements of glucose, or sugar, in the blood. Information from the sensor is sent wirelessly to a device or phone."
These sensors are applied "painlessly to the back of your upper arm and streams glucose readings automatically to your smartphone so you can know your glucose anytime with a quick glance," according to Abbott. The devices end the need for painful, routine finger pricks for calibration and are considered highly accurate.
It was reported that the warning affects about three million sensors in the United States from a single production line. Abbott indicated about half those devices have expired or been used, the CBC stated, adding the company has notified all customers of the problem and has identified and resolved the issue in the affected production lot.
Up to Nov. 14, 2025, Abbott reported 736 serious injuries, and seven deaths associated with this issue. No deaths occurred in the U.S., where 57 injuries were reported.
According to one media report, the company would not share which countries the reported deaths occurred in.
Reason for the Recall.
According to a U.S. Food and Drug Administration (FDA) Early Alert, Abbott stated the certain sensors provide incorrect low glucose readings.
"If undetected, incorrect low glucose readings over an extended period may lead to wrong treatment decisions for people living with diabetes, such as excessive carbohydrate intake or skipping or delaying insulin doses," the FDA release noted. "These decisions may pose serious health risks, including potential injury or death, or other less serious complications."
According to the FDA, Abbott sent all affected customers a letter on Nov. 24, 2025 recommending:
- Patients determine if their current or unused sensor(s) are affected by visiting the FreeStyle Libre website and selecting CONFIRM SENSOR SERIAL NUMBER. Patients need to locate their sensor serial number to determine if the sensor is affected. Those wearing a FreeStyle Libre 3 and FreeStyle Libre 3 Plus sensor can find the serial number in the app or reader. The serial number can also be found on the label on the bottom of the sensor applicator or carton. Those using a sensor with a connected insulin delivery device can refer to the connected insulin delivery device user manual on how to locate the sensor serial number. Visual aids on where to locate the sensor serial numbers can be found in the FDA recall notice.
- Those wearing or have a FreeStyle Libre 3 or FreeStyle Libre 3 Plus sensor that has been confirmed as potentially affected on the FreeStyle Check website or by a customer service representative, should immediately discontinue use and dispose of the affected sensor(s).
- Patients can request a replacement for any potentially affected sensor(s) on the FreeStyle Check website. Select CONFIRM SENSOR SERIAL NUMBER and enter a valid serial number. If the sensor is potentially impacted, patients will be instructed to enter their contact information so a replacement product can be sent at no cost.
- Patients can use a blood glucose meter or the built-in meter in the FreeStyle Libre 3 Reader to make treatment decisions when sensor readings don't match symptoms or expectations.
A sales representative will contact patients with instructions to dispose of impacted samples. Those with additional questions or who need to report any adverse reactions or quality problems experienced with the use of FreeStyle Libre 3 Plus sensors, can call Abbott Customer Service at 1-855-421-6177, Monday to Friday 8 a.m. to 9 p.m. and weekends 9 a.m. to 5 p.m., excluding holidays.
Canadian patients are advised to visit Health Canada's recall website to find the model and serial numbers of the affected products.
What Happens Next?
While it is still too early to know the ultimate impact of the recall on consumers, this is a situation that needs to be monitored, here in Canada and worldwide.
Ensuring the safety of consumer products is the legal responsibility of manufacturers, distributors and sellers who can be held liable for any harm that results. Claims can be brought for damages or injuries related to a product's manufacturing flaws, unsafe design or inadequate warnings.
It is hoped that a person who is harmed due to someone else's negligence would receive justice. Unfortunately, that is not always the case and litigation is sometimes the only path to a solution. Litigation is not solely about seeking compensation for a wrong. It is also a means to force defendants to act more ethically, comply with the law and ensure their products and/or services are safe.
If you or a loved one has been affected by the FreeStyle Libre 3 recall, contact us today. The team of personal injury lawyers at Gluckstein Lawyers have the knowledge, experience and skill to represent you. We are here to answer your questions and address your concerns. Your initial meeting is free and without obligation on your part and we never charge legal fees until your claim is settled.
Gluckstein Lawyers is also interested in hearing from other law firms with clients who have been impacted by recall.